 Multiple Choice Questions
Multiple Choice QuestionsChoose the incorrect statement.
Primary amines show intermolecular hydrogen bonds
Tert-butylamine is a primary amine
Tertiary amines do not show intermolecular hydrogen bonds
Isopropylamine is a secondary amine
Amine that cannot be prepared by Gabriel phthalimide synthesis is
aniline
benzylamine
methylamine
iso-butylamine
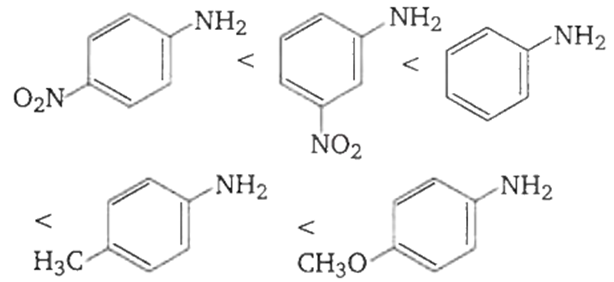
The correct order of increasing basic nature of the following bases is
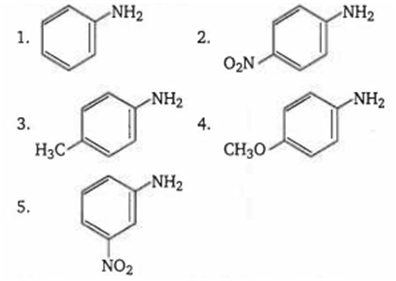
2 < 5 < 1 < 3 < 4
5 < 2 < 1 < 3 < 4
2 < 5 < 1 < 4 < 3
5 < 2 < 1 < 4 < 3
A.
2 < 5 < 1 < 3 < 4
-OCH3 is strongest electron releasing group ( +M effect) which opposes most the dispersion of lone pair of electron of nitrogen into the ring. Thus -OCH, being at para- position imparts highest basicity. NO, being at meta- position stabilises the electron pair of nitrogen only by -I effect. While NO, being present at paraposition due to -M effect and -I effect stabilises the lone pair of electron of nitrogen, most and impart least basicity.
Choose the amide which on reduction with LiAlH4 yields a secondary amine
ethanamide
N-methylethanamide
N, N-dimethylethanamide
phenylmethanamide
Arrange the following amines in the decreasing order of their basic strength. Aniline (I), Benzylamine (II), p-toluidine (III)
I > II > III
II > III > I
III > II > I
II > I > III
Which one of the following compounds will dissolve in an alkali solution after it has undergone reaction with Hinsberg reagent?
CH3NH2
(CH3)3N
(C2H5)2NH
C6H5NHC6H5
Identify the product in the following sequence
3, 4, 5-tribromobenzene
1, 2, 3-tribromobenzene
2, 4, 6-tribromobenzene
3, 4, 5-tribromonitrobenzene
Among the amines
(A) C6H5NH2
(B) CH3NH2
(C) (CH3)3N
(D) (CH3)2NH
A < B < D <C
D < C < B <A
A > B > C >D
B < C < D <A
