 Short Answer Type
Short Answer TypeAn element A has the configuration 1s22s22p63s1 while the configuration of B is 1s22s22p5. What type of bond is likely to be formed between them?
 Long Answer Type
Long Answer TypeWrite the Lewis dot structure of molecule.
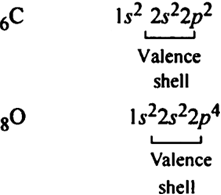
Total number of valence electrons = 4 + 6 = 10
Step II. The skeletal structure of Carbon and Oxygen is CO.
Step III. Putting one shared pair of electrons between C and O and completing the octet on O, the remaining two electrons are the lone pair on C.
Step IV: In the above structure, octet of C is not complete. Hence multiple bonding is required between C and O. Octets of C and O will be complete if there is a triple bond between C and O.![]()
 Short Answer Type
Short Answer TypeWhat do you understand by bond pairs and lone pairs of electrons? Illustrate by giving one example of each type.
