 Long Answer Type
Long Answer Type Short Answer Type
Short Answer Type Long Answer Type
Long Answer TypeName the theory responsible for the definite geometry of covalent molecules. Give main features of the theory ?
 Short Answer Type
Short Answer TypeHow will you explain the following order of the repulsive interactions between pair of electrons on the central atom:
Lone pair - Lone pair > Lone pair - Bond pair > Bond pair - Bond pair.
 Long Answer Type
Long Answer TypeOn the basis of VSEPR theory, discuss the geometry of the following covalent molecules: (i) BeF2 (ii) BF3 (iii) CH4.




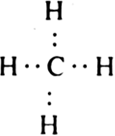

 Short Answer Type
Short Answer TypeAlthough geometries of NH3 and H2O molecules are distorted tetrahedral, the bond angle in water is less than that of ammonia. Discuss.
