 Long Answer Type
Long Answer TypeDiscuss the shape of the following molecules using VSEPR model:
BeCl2, BCl3, SiCl4, AsF5, H2S, PH3
 Short Answer Type
Short Answer Type Long Answer Type
Long Answer TypeDiscuss in brief sp3 hybridisation. Explain the formation of methane and ethane.

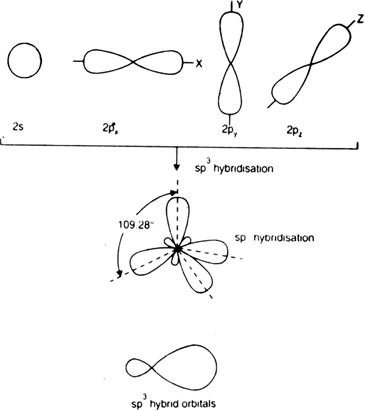

The four C – H bonds are directed towards the four corners of a regular tetrahedron. So methane has a tetrahedral structure. Each H – C – H bond angle is of 109°.28’. Each C – H bond length is 109 pm (1.09 Å).
(ii) Molecular orbital picture of ethane: In ethane molecule, both carbon atoms are in the sp3 hybrid state. In its formation, one hybrid orbital of one carbon atom overlaps with one sp3 hybrid orbital of a second carbon atom along the internuclear axis to form a sigma (σ) C – C bond. The remaining three sp3 hybrid orbitals of each carbon atom overlap with 1 s orbital of hydrogen atom axially to form six sigma C – H bonds.

The length of C-C bond in ethane is 154 pm (or 1- 54 Å) and that of each C - H bond is 109 pm (or 1-09 Å).
Discuss in brief sp2 hybridization (hybridization in C = C bond). Discuss the molecular orbital structure of ethylene (first member of alkene).
Or
Draw diagrams showing the formation of a double bond between carbon atoms in C2H4.
 Short Answer Type
Short Answer TypeApart from tetrahedral geometry, another possible geometry for CH4 is square planar with four H atoms at the corners of the square and the C atom at its centre. Explain why CH4 is not square planar?
 Long Answer Type
Long Answer Type Short Answer Type
Short Answer TypeThe central atoms in CH4, NH3 and H2O are all said to have similar hybridisation but the bond angle H – A – H (where A is C, N or O) is different in each case. Explain stating in which case it is maximum and in which case it is minimum.
