 Long Answer Type
Long Answer TypeDiscuss the formation of N2 molecule on the basis of MO theory. Predict its:
(i) Bond order
(ii) Magnetic character.
Write the stability configuration of ![]() and predict their bond order, stability and magnetic character.
and predict their bond order, stability and magnetic character.
Draw the molecular orbital energy diagram for oxygen molecule (O2) and show that:
(i) It has a double bond
(ii) It has paramagnetic character.
Using a molecular orbital diagram, predict the bond order, stability and magnetic character of O2–,O2+, and  . Also, write their electronic configurations.
. Also, write their electronic configurations.
O2–(Superoxide ion): This ion is formed by the addition of one electron.
O2 + e- → O2
This additional electron will be added up in the  molecular orbital.
molecular orbital.
Electronic configuration:
Bond order:
Here Nb = 8; Na = 5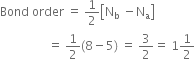
Stability : As the bond order is positive, it is quite stable.
Magnetic character: It has one unpaired electron in the  molecular orbital.
molecular orbital.
therefore, it is paramagnetic.
O+2 ion This ion is formed by the loss of one electron from O2 molecule.
O2 →O2+ e–
This electron will be lost from  *2px or
*2px or  *2py molecular orbital.
*2py molecular orbital.
Electronic configuration: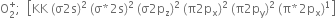
Bond order: Here Nb = 8; Na = 3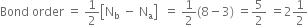
Stability: As the bond order is positive, it is quite stable.
Magnetic character: Since 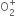 ion has one unpaired electron in the
ion has one unpaired electron in the  orbital, therefore, it is paramagnetic.
orbital, therefore, it is paramagnetic. : The ion is formed when O2 molecule gains two electrons
: The ion is formed when O2 molecule gains two electrons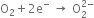
These two electrons will be added to the  *2px and
*2px and  *2py molecular orbitals, one in each.
*2py molecular orbitals, one in each.
Electronic configuration:
Bond order: Here Nb = 8; Na = 6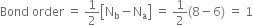
Stability: As the bond order is positive, it is quite stable.
Magnetic character: As one of the molecular orbital has a unpaired electron, it is diamagnetic.
Discuss the relatives stabilities, bond dissociation energies and bond lengths of O2,  species.
species.
Is the bond order in superoxide ion more or less than in peroxide ion? Explain on the basis of MO theory.
 Short Answer Type
Short Answer TypeUsing bond order show that N2 would be expected to have a triple bond, F2 a single bond and Ne2 no bond.
Show that N2 molecule has a greater bond dissociation energy than N-2 where O2 has lower bond dissociation energy than O+2.
