 Multiple Choice Questions
Multiple Choice QuestionsAcetylene molecule has carbon in:
sp -hybridization
sp2 -hybridization
sp3 -hybridization
sp3d -hybridization
In which of the following compound sp2 hybridisation is absent ?
CH ≡ C - CH = CH2
CH ≡ C - CH2 - CH3
CH3 - CH = CH2
CH2 = CH - CH2 - CH3
C - Cl bond is stronger than C - I bond because
C - Cl bond is more ionic than C - I
C - Cl bond is more ionic than C - I
C -Cl bond is more covalent than C - I
C - Cl bond length is longer than C - I
Bond energy of hydrogen gas is - 433 kJ. How much is the bond dissociation energy of 0.5 mole of hydrogen gas ?
-433 kJ
+433 kJ
-216 kJ
+216 kJ
Geometry of ammonia molecule and the hybridisation of nitrogen involved in it are
sp3-hybridisation and tetrahedral geometry
sp3-hybridisation and distorted tetrahedral geometry
sp2-hybridisation and triangular geometry
None of the above
Be in BeCl2 undergoes
diagonal hybridisation
trigonal hybridisation
tetrahedral hybridisation
no hybridisation
In 1- butene number of σ-bonds is
8
10
11
12
C.
11
First bond between any two atoms is σ bond and rest are bonds. In the structure of 1-butene, there are 11 σ bonds. It can be represented as-
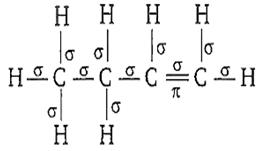
The catenation tendency of C, Si and Ge is in the order Ge < Si < C. The bond energies (in kJ mol-1) of C-C, Si-Si and Ge-Ge bonds, respectively are
167, 180, 348
180, 167, 348
348, 167, 180
348, 180, 167
