 Multiple Choice Questions
Multiple Choice QuestionsWhich of the following statements is true?
Hybridisation of the central atom in NH3 and CH4 is sp2
BeCl2 has V shape while SO2 is linear
SF6 is octahedral and F-S-F bond angle is 90°
CO2 has dipole moment
Bond dissociation energies of HF, HCl, HBr follow the order
HCl > HBr > HF
HF > HBr > HCl
HF > HCl > HBr
HBr > HCl > HF
Which one ofthe following is a correct set?
H2O, sp3, angular
H2O, sp2, linear
NH4+, dsp2,square planar
CH4, dsp2, tetrahedral
The bond energies (in kJ mol-1) of P-H; As- H and N-H are respectively
247, 389 and 318
247, 389 and 318
318, 389 and 247
318, 247 and 389
The calculated magnetic moment (in Bohr magnetons of Cu2+ ion is)
1.73
zero
2.6
3.4
A.
1.73
Electronic configuration of Cu2+ is-
1s2, 2s2, 2p6, 3s2, 3p6, 3d9 (because 2e- goes from 4s orbital)
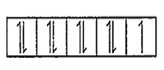
Number of unpaired electrons = 1
Magnetic moment =
=
=
= 1.732
Therefore, 1.732 is the magnetic moment of Cu2+.
Which one of the following molecules contain both ionic and covalent bonds?
CH2Cl2
K2SO4
BeCl2
SO2
What is the hybridization state of the central atom in the conjugate base of NH ion?
sp
sp3
sp2
dsp2
If the bond length and dipole moment of a diatomic molecule are 1.25 A and 1.0 D respectively, what is the percent ionic character of the bond ?
10.66
12.33
16.66
19.33
