 Multiple Choice Questions
Multiple Choice QuestionsIf the value of bond order is zero, then
molecule will be stable
molecule will be unstable
molecule will be in ionic state
None of the above
Which of the following is a proper match?
| Shape | |
| A. 0.115 - 0.225 | i. Triangular |
| B. 0.225 - 0.414 | ii. Tetrahedral |
| C. 0.414 - 0.732 | iii. Cubic |
| D. 0.732 - 1 | iv. Octahedral |
A - i; B - ii; C - iv; D - iii
A - iii; B - ii; C - iv; D - i
A - i; B - iii; C - ii; D - iv
A - ii; B - iv; C - i; D - iii
According to MOT which of the following is correct for potassium hexa cyano ferrate (III)?
It is octahedral complex.
t2g orbital contains the e- of metal.
For iron, overlapping between empty orbitals and ligand orbitals takes place.
All of the above
Which of the following is an electron deficient compound?
B2H6
NH3
C2H6
CCl4
A.
B2H6
B2H6 is an electron deficient compound in which B is sp3-hybridised state. It has the following H-bridged structure.
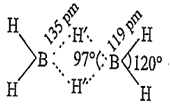
The shape of water molecule according to VSEPR theory, is
octahedral
distorted tetrahedral
trigonal planar
trigonal bipyramidal
