Multiple Choice Questions
Multiple Choice QuestionsDuring the formation of a chemical bond
electron-electron repulsion becomes more than the nucleus-electron attraction.
energy of the system does not change.
energy increases
energy decreases
In acetylene molecule, between the carbon atoms there are
three pi bonds
one sigma and two pi bonds
two sigma and one pi bonds
three sigma bonds
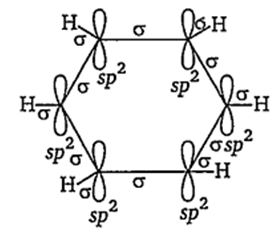
The overlapping of orbitals in benzene is of the type
sp-sp
p-p
sp2-sp2
sp3-sp3
C.
sp2-sp2
The molecular orbital picture of benzene shows that in it all the six carbon atoms are sp2 hybridised. Out of these three sp2 hybrid orbitals of each C atom, two orbitals overlap with sp2 hybrid orbitals of adjacent C atoms to form six C—C single bonds. The remaining sporbital of each C atom overlaps with s-orbital of each hydrogen atom to form six C—H single sigma bonds. Each C atom is now left with one unhybridised p-orbital perpendicular to the plane of the ring.