 Multiple Choice Questions
Multiple Choice QuestionsConsider the following ions:
(i) Ni2+ (ii) Co2+ (iii) Cr2+ (iv) Fe3+
(Given, at. no. Cr = 24, Fe = 26, Co = 27, Ni = 28)
The correct order of magnetic moment of these ions is
(i) < (ii) < (iii) < (iv)
(iv) < (ii) < (iii) < (i)
(i) < (iii) < (ii) < (iv)
(iii) < (iv) < (ii) < (i)
Geometry of methyl free radical is
pyramidal
planar
tetrahedral
linear
B.
planar
Number of hybrid orbitals = number of σ bonds + number of lps.
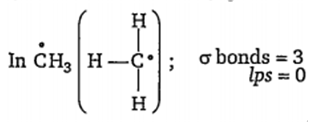
Number of hybrid orbitals =3 + 0 =3
Hence, hybridisation is sp2 and geometry is planar. Methyl free radical is highly reactive due to the presence of one unpaired electron.
The electron configuration of the oxide ion is much most similar to the electron configuration of the
sulphide ion
nitride ion
oxygen atom
nitrogen atom
