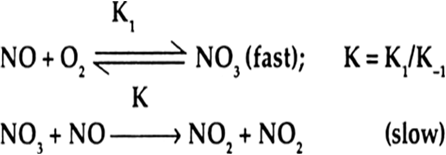122.
Write the overall reaction and the rate law from the following elementary reactions:
What is the order of the reaction?
For the given reaction, the overall reaction will be:
____________________________________
____________________________________
Elementary step NOBr2 + NO → 2NOBr is slow, thus it is rate determining step. Its rate is given by
...(i)
For elementary reversible step
the equilibrium constant k is given by
or ...(ii)
Putting the expression for [NOBr2] from equation (ii) in to equation (i) we shall get the rate law equation as
where k = k' k = rate constant of the overall reaction. From the rate equation it is formed that the order with respect to NO is 2 with respect to the order is 1 and overall order of reaction is
2 + 1 = 3.
130 Views
 Short Answer Type
Short Answer Type Long Answer Type
Long Answer Type Short Answer Type
Short Answer Type Long Answer Type
Long Answer Type