 Short Answer Type
Short Answer TypeWhy are cimetidine and ranitidine better antacids than sodium hydrogencarbonate or magnesium or aluminium hydroxide?
Synthetic detergents can be used for washing purposes even when the water is hard, whereas soaps are not suitable for washing with hard water. This is because of the fact that synthetic detergents can lather with hard water. This is because of the fact that synthetic detergents can lather well even in hard water because they do not form insoluble calcium or magnesium salts on reacting with calcium and magnesium ions present in hard water. Soap, however, forms insoluble calcium and magnesium salts with hard water which reduces its cleansing ability.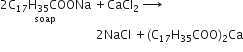
Hence, Synthetic detergents better than soaps.
 Long Answer Type
Long Answer TypeExplain the following terms with suitable examples:
(i) cationic detergents,
(ii) anionic detergents and
(iii) neutral detergents.
 Short Answer Type
Short Answer Type