 Long Answer Type
Long Answer TypeWhat is long form of the periodic table? Discuss in brief its salient structural features.
 Short Answer Type
Short Answer Type Long Answer Type
Long Answer Type Short Answer Type
Short Answer Type Long Answer Type
Long Answer Type Short Answer Type
Short Answer Type Long Answer Type
Long Answer TypeThe long form of periodic table has been divided into four blocks. These are called:
(i) s-block (ii) p-block
(iii) d-block , (iv) f-block.
Division of elements into s, p, d and f-blocks: This division is based upon the name of the orbital which receives the last electron.
1. s-block elements. The elements in which the last electron enters the s-orbital of the outermost enthalpy level are called .s-block elements. The general electronic configuration of s-block elements is ns1-2 where n stands for the outermost shell. In s-block, the alkali metals of group 1 and alkaline earth metals of group 2 are included.
2. p-block elements: The elements in which the last electron enters the p-orbital of the outermost enthalpy level are called p-block elements. The general electronic configuration of p-block elements in ns2np1-6 where n stands for the outermost shell. The elements of Groups 13, 14, 15, 16, 17, 18 having 3, 4, 5, 6, 7 and 8 electrons respectively in the outermost enthalpy levels constitute p-block elements.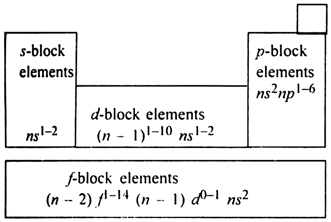
3. d-block elements:The elements in which the last electron enters the d-orbital of the penultimate enthalpy level (last but one shell) are called d-block elements. General electronic configuration of d-block elements is (n - 1) d1-10 ns1-2 where n represents the valence shell, d-block elements have three complete series of ten elements in each whereas the fourth series is incomplete.
Note, Exception is 46Pd whose configuration is 4d10 550:
4. f-block elements:The elements in which the last electron enters the f-orbital of the anti-penultimate (third to the outermost shell) enthalpy level are called f-block elements. The general electronic configuration of f-block elements is (n -2) f1-14 (n-1) d0-1 ns2where n represents the outermost shell. Such type of elements have two series. First is 4f series which is also called lanthanoides series whereas second is 5f series which is also described as actinoide series. These two series are placed separately at the bottom of the periodic table. The elements included in these two series are called inner transition elements.
 Short Answer Type
Short Answer TypeWhat are the advantages of classifying the elements into s, p, d and f-block elements?
Which element do you think would have been named by:
(i) Lawrence Berkeley Laboratory
(ii) Seaborg's group?
