 Short Answer Type
Short Answer TypeThe hexaquo manganese(II) ion contains five unpaired electrons, while the hexacynoion contains only one unpaired electron. Explain using crystal field theory.
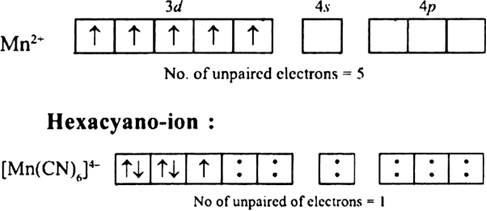
Hence, hexaaquo manganese (ll) ion has five unpaired electrons, while hexacyano ion has only one unpaired electron.
Identify the ligands from the following coordination compounds:
(a) [Co(en)2Cl(NO2)2]
(b) K[Co(CN)(CO2)(NO)]
(c) K3[Al(OH)6]
(d) [Co(H2O)2(NH3)4](OH)3
Provide systematic names to the following complexes:
(i) [Fe(H2O)5(NO)]SO4,
(ii) [Cr(NH3)6]3+
(iii) [Pt(NH3)2Cl2],
(iv) [NiCl4]2–
