 Long Answer Type
Long Answer Type
[Ni(CN)4]2– : Outer electronic configuration of nickel (Z = 28) in ground state is 3d 84s2. Nickel in this complex is in the +2 oxidation states. It achieves +2 oxidation state by the loss of the two 4s- electrons. The resulting of Ni2+ ion has outer electronic configuration of 3d8. The two unpaired 3d- electrons are forced to pair up.
The resulting vacant 3d-orbital along with the 4s-orbital and two 4p-orbitals hybridised to give four equivalent dsp2 hybridised orbitals. Four pairs of electrons, one from each cyanide ion occupy the four vacant hybrid orbitals so produced. Therefore, it is diamagnetic because there is no unpaired electrons.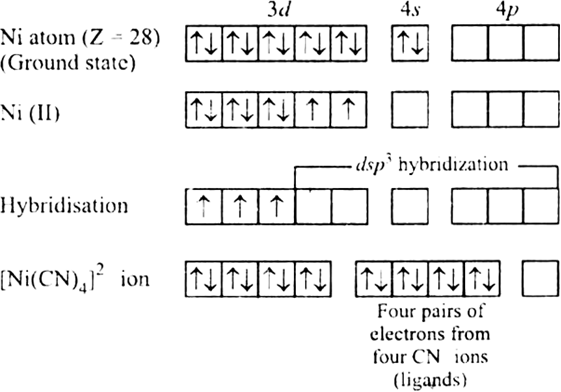
 Short Answer Type
Short Answer TypeGive the oxidation state, d-orbital occuptaion and coordination number of the central metal ion in the following complexes:
K3[Co(C2O4)3]
Give the oxidation state, d-orbital occuptaion and coordination number of the central metal ion in the following complexes:
cis-[Cr(en) 2Cl2]Cl
Give the oxidation state, d-orbital occuptaion and coordination number of the central metal ion in the following complexes:
(NH4)2[CoF4]
Give the oxidation state, d-orbital occuptaion and coordination number of the central metal ion in the following complexes:
[Mn(H2O)6SO4
Write down the IUPAC name for each of the following complexes and indicate the oxidation state, electronic configuration & coordination number, Also give stereochemistry and magnetic moment of the complex:
K[Cr(H2O)2(C2O4)2]. 3H2O
Write down the IUPAC name for each of the following complexes and indicate the oxidation state, electronic configuration & coordination number, Also give stereochemistry and magnetic moment of the complex:
CrCl3(py)3
Write down the IUPAC name for each of the following complexes and indicate the oxidation state, electronic configuration & coordination number, Also give stereochemistry and magnetic moment of the complex:
Cs[FeCl4].
