 Short Answer Type
Short Answer TypeExplain the following:
[Ni(H2,O)6]2+ turns blue when changed to [Ni(NH3)6]3+ by adding NH3
Explain the following:
Violet coloured [Cr(H2O)6]3+ becomes bright blue when reduced to [Cr(H2O)6]2+
 Long Answer Type
Long Answer Type Short Answer Type
Short Answer TypeGive the number of unpaired electrons in the following complex ions:
[FeF6]4– and [Fe(CN)6]4–
Name the isomerism exhibited by the following pair of coordination compounds:
[Co(NH3)5Br]SO4 and [Co(NH3)5SO4] Br
The atoms or molecules or ions which donate pair of electrons to the central metal atom and thus forms coordinate bond with the central metal atoms are called ligands.
Example of a bidentate ligand: Ethylene diamine.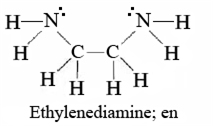
 Long Answer Type
Long Answer TypeExplain as to how the two complexes of nickel, [Ni(CN)4]2– and Ni(CO)4, have different structures but do not differ in their magnetic behaviour (Ni = 28).
