Short Answer Type
Short Answer TypeExplain the following:
[Ni(H2,O)6]2+ turns blue when changed to [Ni(NH3)6]3+ by adding NH3
Explain the following:
Violet coloured [Cr(H2O)6]3+ becomes bright blue when reduced to [Cr(H2O)6]2+
 Long Answer Type
Long Answer Type Short Answer Type
Short Answer TypeGive the number of unpaired electrons in the following complex ions:
[FeF6]4– and [Fe(CN)6]4–
Name the isomerism exhibited by the following pair of coordination compounds:
[Co(NH3)5Br]SO4 and [Co(NH3)5SO4] Br
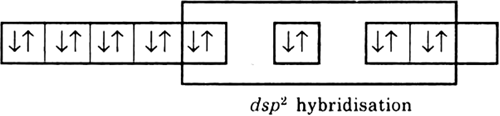
 Long Answer Type
Long Answer TypeExplain as to how the two complexes of nickel, [Ni(CN)4]2– and Ni(CO)4, have different structures but do not differ in their magnetic behaviour (Ni = 28).