 Short Answer Type
Short Answer Type Long Answer Type
Long Answer TypeDraw the structures of geometrical isomers of the following coordination complexes:
[Co(NH3)3Cl3] and [CoCl2(en)2+]
(en = ethylene diamine and atomic number of Co is 27).
Geometrical isomerism:
This type of isomerism arises in heteroleptic
complexes due to different possible geometric
arrangements of the ligands. Important examples of this behaviour are found with coordination numbers 4 and 6. In a square planar complex of formula [MX2L2] (X and L are unidentate), the two ligands X may be arranged adjacent to each other in a cis isomer, or opposite to each other in a trans isomer. for example geometrical isomers of Pt[(NH3)2Cl2](3).png)
Each geometrical isomer has a central platinum surrounded by the same four ligands (2 chloro and 2 amine in each case) which lie at the corners of a square. But they differ in the special positions of the ligands. In cis-isomer two similar ligands occupy positions at 90° to one another. In trans-isomer two similar ligands occupy positions opposite to one another at 180° apart.
Optical isomerism: When the coordination compounds have similar formula but differ in their abilities to rotate directions of the plane of the polarized light, they are said to exhibit optical isomerism and the molecules are optical isomers. The optical isomers are pair of molecules which are non-super imposabie mirror images of each other. For example, cis-dichlorobis (ethylene diamine) cobalt(II) ion exhibits optical activity.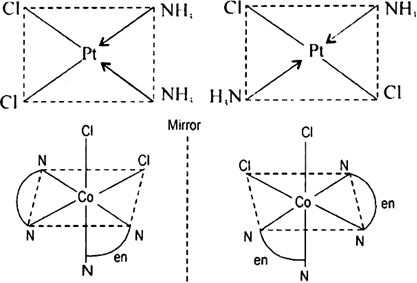
Fig. Cis [Co(en)2Cl2,]+ and its mirror image.
One form of cis[Co(en)2Cl2] which rotates plane of polarized light in right direction is called dextro isomer (or d-form or + isomer). The other isomer which rotates the plane of polarised light in left direction is designated laevo-isomer (or l or-isomer).
 Short Answer Type
Short Answer Type Long Answer Type
Long Answer Type Short Answer Type
Short Answer Type Long Answer Type
Long Answer Type(i) Explain geometrical isomerism with reference to square planar complexes giving one example. How is tetrahedral complexes with simple ligands do not exhibit geometrical isomerism?
(ii) Using valence bond theory, predict the shape and magnetism (paramagnetism) or diamagnetism of [Co(CO)4]– (at. no. of Co = 27)?
(iii) How is stability of coordination compounds determined in aqueous solution?
 Fill In the Blanks
Fill In the Blanks