 Short Answer Type
Short Answer TypeIn a geometry of [PtCl4]2– is square planar, what orbitals of platinum are involved in the bonding?
The electronic configuration of Platinum is [Xe] 4f14 5d9 6s1
Or [Xe] 4f14 5d8 6s2
Oxidation of Pt in this complex is +2 thus, 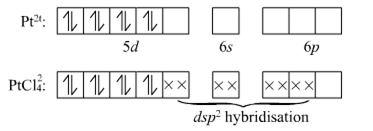
The four chlorine atom filled the empty orbital. Therefore the the hybridziation of [PtCl4]2– is dsp2.
Complex should be tetrahedral instead of square planar theoretically. But the size of Pt is large that it forms strong bond with ligand. Due to which strong repulsion between the electron of Pt and ligand takes place which result in strong crystal field splitting. The strong field splitting breaks the degeneracy of dx2- y2 and dz2 orbital. Hence stabilizes the square planer arrangement more than tetrahedral thus it should be square planar.
