 Short Answer Type
Short Answer TypeThe valence shell electronic configuration of ground state Ni atom is 3d8 4s2.
All of these 10 electrons are pushed into 3d orbitals and get paired up when strong field CO ligands approach Ni atom. The empty 4s and three 4p orbitals undergo sp3 hybridization and form bonds with CO ligands to give Ni(CO)4. Thus Ni(CO)4 is diamagnetic.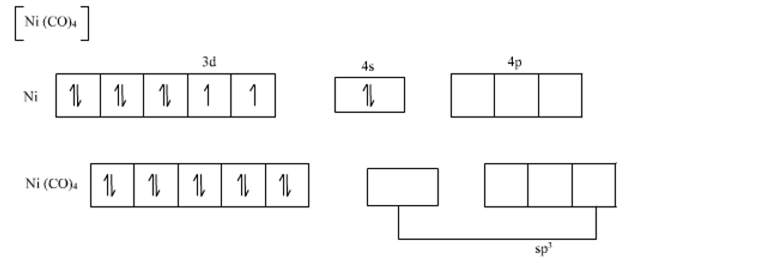
in NiCl42-, there is Ni2+ ion, However, in presence of weak field Cl- ligands, no pairing of d-electrons occurs. Therefore, Ni2+ undergoes sp3 hybridization to make bonds with Cl- ligands in tetrahedral geometry. As there are unpaired electrons in the d-orbitals, NiCl42- is paramagnetic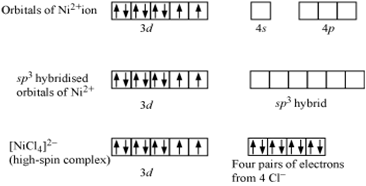
Since (NiCl4)2– is paramagnetic thus it have more magnetic moment.
What do you understand by crystal field splitting? Draw figure to show splitting of degenerate d-orbitals in an octahedral crystal field.
What are the main postulates of Werner’s theory? Write the correct formula and IUPAC name of the coordination compound for CrCl3.6H2O, one mole of which give two moles of AgCl with AgNO3,.
 Long Answer Type
Long Answer TypeGive the systematic name for each of the following compounds:
(i)[Pt(NH3)4Cl2][PtCl4]
(ii) [Cr(CO)5Cl](ClO3)2
(iii) K3[Cr(CN)6]
(iv)[Co(NH)4Cl2]+
(v) (NH4)3[Co(NO2)6]
(vi) K2[CuCl4]
Give the chemical formula for each of the following compounds:
(i) Potassium hexanitro cobaltate(III).
(ii) Potassium hexacyano cobaltate(III).
(iii) Ammonium trans-dichlorodiodo aurate(III).
(iv) Sodium pentacyano carbonyl ferrate(II).
(v) Diamminechlorido(methylamine)plantinum(II)
chloride.
(vi)Dichloridobis(ethane–1,2diamine)platinum(IV) nitrate
 Short Answer Type
Short Answer Type