 Short Answer Type
Short Answer TypeWhat do you understand by crystal field splitting? Draw figure to show splitting of degenerate d-orbitals in an octahedral crystal field.
The crystal field splitting, depends upon the field produced by the ligand and charge on the metal ion. Some ligands are able to produce strong fields in which case, the splitting will be large whereas others produce weak fields and consequently result in small splitting of d orbitals. Therefore the d orbital will be degenrate in two set such as t2g and eg orbital .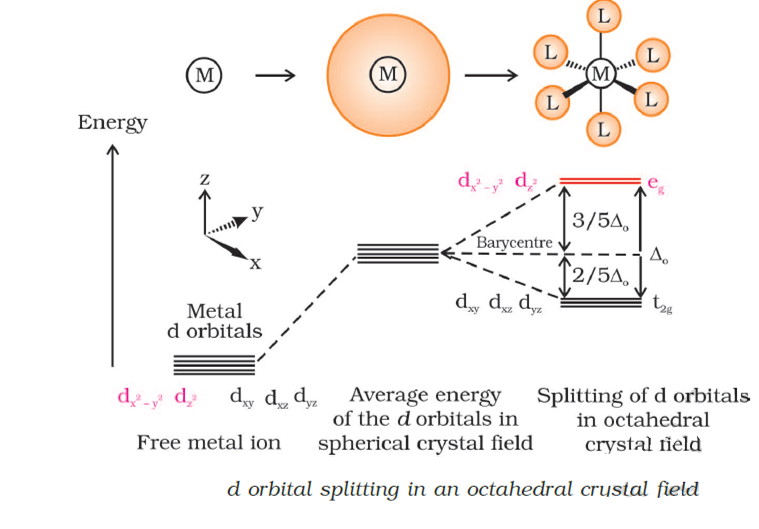
What are the main postulates of Werner’s theory? Write the correct formula and IUPAC name of the coordination compound for CrCl3.6H2O, one mole of which give two moles of AgCl with AgNO3,.
 Long Answer Type
Long Answer TypeGive the systematic name for each of the following compounds:
(i)[Pt(NH3)4Cl2][PtCl4]
(ii) [Cr(CO)5Cl](ClO3)2
(iii) K3[Cr(CN)6]
(iv)[Co(NH)4Cl2]+
(v) (NH4)3[Co(NO2)6]
(vi) K2[CuCl4]
Give the chemical formula for each of the following compounds:
(i) Potassium hexanitro cobaltate(III).
(ii) Potassium hexacyano cobaltate(III).
(iii) Ammonium trans-dichlorodiodo aurate(III).
(iv) Sodium pentacyano carbonyl ferrate(II).
(v) Diamminechlorido(methylamine)plantinum(II)
chloride.
(vi)Dichloridobis(ethane–1,2diamine)platinum(IV) nitrate
 Short Answer Type
Short Answer Type