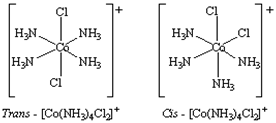289.
Illustrate with an example each of the following terms: (i) Ionization isomerism, (ii) coordination isomerism, (iii) Linkage isomerism, (iv) Geometrical isomerism (v) Optical isomerism.
i) Ionization isomers: Ionization isomers are identical except for a ligand has exchanging places with an anion or neutral molecule that was originally outside the coordination complex. The central ion and the other ligands are identical. For example the two compounds, pentaamminebromocobalt sulfate, [CoBr(NH3)5]SO4, and pentaamminesulfatocobalt bromide, [Co(SO4)(NH3)5]Br.
ii) Coordination isomers: This type of isomerism arises from the interchange of ligands between cationic and anionic entities of different metal ions present in a complex. An example is provided by [Co(NH
3)
6][Cr(CN)
6], in which the NH
3 ligands are bound to Co
3+ and the CN– ligands to Cr
3+. In its coordination isomer [Cr(NH
3)
6][Co(CN)
6], the NH
3 ligands are bound to Cr
3+ and the CN
– ligands to Co
3+.
iii) Linkage isomerism: Linkage isomerism arises in a coordination compound containing ambidentate ligand. A simple example is provided by complexes containing the thiocyanate ligand, NCS–, which may bind through the nitrogen to give M–NCS or through sulphur to give M–SCN. For example [Co(NH
3)
5(NO
2)]Cl
2, which is obtained as the red form, in which the nitrite ligand is bound through oxygen (–ONO), and as the yellow form, in which the nitrite ligand is bound through nitrogen (–NO
2).
iv) Geometrical Isomerism: Geometrical isomerism arises in heteroleptic complexes due to different possible geometric arrangements of the ligands. Important examples of this behaviour are found with coordination numbers 4 and 6. In a square planar complex of formula [MX
2L
2] (X and L are unidentate), the two ligands X may be arranged adjacent to each other in a cis isomer, or opposite to each other in a trans. For example Cis -trans isomer of [Co(NH
3)
4Cl
2]
+.
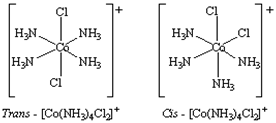
v) Optical isomerism: Optical isomerism are mirror images that cannot be superimposed on one another. These are called as enantiomers. The molecules or ions that cannot be superimposed are called chiral. The two forms are called dextro (d) and laevo (l) depending upon the direction they rotate the plane of polarised light. For example [Co(en)
3]
3+.
324 Views
 Short Answer Type
Short Answer Type Long Answer Type
Long Answer Type