 Multiple Choice Questions
Multiple Choice QuestionsWhich of the following processes does not involve oxidation of iron?
Rusting of iron sheets
Decolourisation of blue CuSO4 solution by iron
Formation of Fe(CO)5 from Fe
Formation of Fe(CO)5 from Fe
Which of these statements about [Co(CN)6]3- is true?
[Co(CN)6]3- has no unpaired electrons and will be in a low-spin configuration.
[Co(CN)6]3- has four unpaired electrons and will be in a low-spin configuration
[Co(CN)6]3- has four unpaired electrons and will be in high-spin configuration.
[Co(CN)6]3- has four unpaired electrons and will be in high-spin configuration.
Cobalt (III) chloride forms several octahedral complexes with ammonia, which of the following will not give a test for chloride ions with silver nitrated at 250 C?
CoCl. 3NH3
CoCl. 4NH3
CoCl. 5NH3
CoCl. 5NH3
Among the following complexes, the one which shows zero crystal field stabilisation energy (CFSE) is
[Mn(H2O)6]3+
[Fe(H2O)6]3+
[Co(H2O)6]3+
[Co(H2O)6]3+
Which of the following complexes is used to be as an anticancer agent ?
mer=[Co(NH3)Cl3]
cis-[PtCl2(NH3)2]
cis-K2[PtCl2Br2]
cis-K2[PtCl2Br2]
Which of the following compounds will undergo racemization when a solution of KOH hydrolysis?
(i) and (ii)
(iv)
(iii) and (iv)
(iii) and (iv)
(iii) and (iv)
A magnetic moment of 1.73 BM will be shown by one among the following
[Cu(NH3)4]2+
[Ni(CN)4]2-
TiCl4
TiCl4
An excess of AgNO3 is added to 100 mL of a 0.01 M solution of dichlorotetraaquachromium (III) chloride. The number of moles of AgCl precipitate would be
0.001
0.002
0.003
0.003
Which of the following acids does not exhibit optical isomerism?
Maleic acid
α-amino acids
Lactic acid
Lactic acid
A.
Maleic acid
Only those compounds exhibit optical isomerism, which has chiral centre and / Or absence of symmetry elements. (chiral carbon is the four valencies of which are satisfied by four different groups.)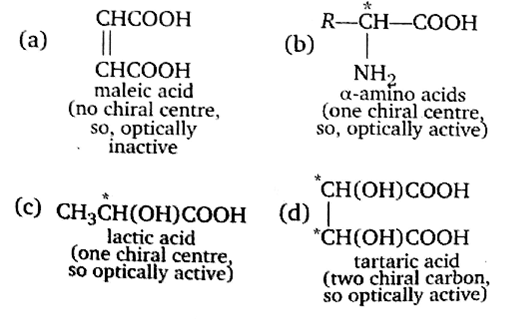
Thus, maleic acid does not exhibit optical isomerism.
