 Multiple Choice Questions
Multiple Choice QuestionsOut of TiF62-, CoF63-, Cu2Cl2 and NiCl42- (Z of Ti = 22, Co= 27, Cu = 29, Ni =28) the colourless species are
TiF62- and CoF63-
Cu2Cl2 and NiCl42-
TiF62- and Cu2Cl2
TiF62- and Cu2Cl2
Which of the following compound will exhibit cis-trans (geometrical) isomerism?
2-butene
Butanol
2-butyne
2-butyne
A.
2-butene
Compounds which have at least one double bond (C=C) and the groups attached with double bonded carbon atoms are different exhibit geometrical isomerism.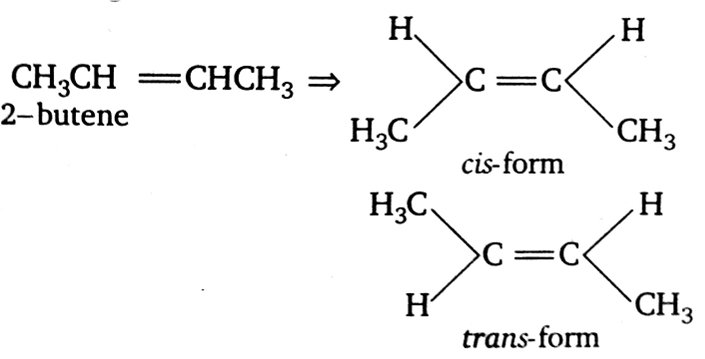
On the basis of the following Eo values; the strongest oxidising agent is
[Fe(CN)6]4- → [Fe(CN)6]3-] +e- ;
Eo = -0.35 V
Fe2+ → Fe3+ +e-; E = -0.77 V
[Fe(CN)6]4-
Fe2+
Fe3+
Fe3+
Which of the following complexes exhibits the highest paramagnetic behaviour?
Where gly = glycine, en = ethylenediamine and bpy = bipyridyl moities
(At. no. ; Ti = 22, V = 23, Fe =26, Co = 27)
[V(gly)2 (OH)2 (NH3)2]+
[Fe(en)(bpy)(NH3)2]+
[CO(OX)2(OH)2]-
[CO(OX)2(OH)2]-
In which of the following coordination entities the magnitude of Δo (CFSE in the octahedral field) will be maximum?
(Atomic number Co = 27)
[Co(H2O)6]3+
[CO(NH3)6]3+
[CO(CN)6]3-
[CO(CN)6]3-
Which of the following will give a pair of enantiomorphs?
(en = NH2CH2CH2NH2)
[Co(NH3)Cl2]NO2
[Cr(NH3)6]Co(CN)6]
[Co(en)2Cl2]Cl
[Co(en)2Cl2]Cl
If there is no rotation of plane polarises light by a compound in a specific solvent, thought to by chiral, it may mean that:
the compound is certainly a chiral
the compound is certainly meso
there is no compound in the solvent
there is no compound in the solvent
CH3-CHCl-CH2-CH3 has a chiral centre. Which one of the following represents its R configuration?




The d-electron configuration of Cr3+, Mn2+, Fe2+ and Ni2+ are 3d4, 3d5, 3d6 and 3d8 respectively. Which one of the following aqua complexes will exhibit the minimum paramagnetic behaviour?
(At. no. Cr = 24, Mn = 25, Fe = 26, Ni = 28)
[Mn(H2O)6]2+
[Fe(H2O)6]2+
[Ni(H2O)6]2+
[Ni(H2O)6]2+
