 Short Answer Type
Short Answer TypeFollowing reactions occur at cathode during the electrolysis of aqueous silver chloride solution:
Ag+ (aq) + e- → Ag(s) E° = +0.80 V
H+ (aq) + e- → 1/2 H2 (g) E° = 0.00 V
On the basis of their standard reduction electrode potential (E°) values, which reaction is feasible at the cathode and why?
Define limiting molar conductivity. Why conductivity of an electrolyte solution decreases with the decrease in concentration?
Calculate emf of the following cell at 25 °C:
Fe | Fe2+(0.001 M) || H+ (0.01 M) | H2 (g) (1 bar) | Pt(s)
E°(Fe2+ | Fe) = –0.44 V E°(H+ | H2 ) = 0.00 V
The resistance of a conductivity cell filled with 0.1 mol L-1 KCl solution is 100 . If the resistance of the same cell when filled with 0.02 mol L-1 KCl solution is 520 , calculate the conductivity and molar conductivity of 0.02 mol L-1 KCl solution. The conductivity of 0.1 mol L-1 KCl solution is 1.29x 10-2
, calculate the conductivity and molar conductivity of 0.02 mol L-1 KCl solution. The conductivity of 0.1 mol L-1 KCl solution is 1.29x 10-2 -1cm-1
-1cm-1
State Faraday's first law of electrolysis. How much charge in terms of Faraday is required for the reduction of 1 mol of Cu2+ to Cu.
Calculate emf of the following cell at 298 K: Mg(s) | Mg2+(0.1 M) || Cu2+ (0.01) | Cu(s)
[Given E0 cell = +2.71 V, 1 F = 96500 C mol-1]
The cell reaction can be represented as:
Mg(s) + Cu2+(aq.) ---> Mg+(aq.) + Cu(s)
Given:
 =+2.71 V
=+2.71 V
T = 298 K
According to the Nernst equation:
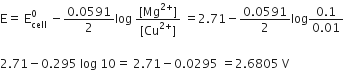
=2.71-0.0295 log 10 = 2.71-0.0295
=2.6805 V
The conductivity of 0.20 M solution of KCl at 298 K is 0.025 S cm-1. Calculate its molar conductivity?
a) What type of a battery is the lead storage battery? Write the anode and the cathode reactions and the overall reaction occurring in a lead storage battery when current is drawn from it.
(b) In the button cell, widely used in watches, the following reaction takes place
Determine E° and G° for the reaction 
