 Multiple Choice Questions
Multiple Choice Questions| Electrolyte | KCl | KNO3 | HCl | NaOAc | NaCl |
| ∧∞S cm2 mol- | 149.9 | 145.0 | 426.2 | 91.0 | 126.5 |
517.2
552.7
390.7
390.7
Calomel (Hg2Cl2) on reaction with ammonium hydroxide gives
HgNH2Cl
NH2 – Hg – Hg – Cl
Hg2O
Hg2O
Which among the following factors is the most important in making fluorine the strongest oxidizing halogen?
Electron affinity
Bond dissociation energy
Hydration enthalpy
Hydration enthalpy
In hydrogen-oxygen fuel cell, combustion of hydrogen occurs to
generate heat
remove adsorbed oxygen from electrode surfaces
produce high purity water
produce high purity water
Consider the following E° values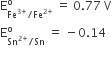
Under standard conditions the potential for the reaction
Sn(s) + 2Fe3+(aq) → 2Fe2+(aq) + Sn2+(aq) is
1.68 V
0.63 V
0.91 V
0.91 V
The standard e.m.f of a cell, involving one electron change is found to be 0.591 V at 25°C. The equilibrium constant of the reaction is (F = 96,500 C mol-1: R = 8.314 JK-1 mol-1)
1.0×101
1.0×1030
1.0×1030
1.0×1030
The limiting molar conductivities Λ° for NaCl, KBr and KCl are 126, 152 and 150 S cm2 mol-1 respectively. The Λ° for NaBr is
128 S cm2 mol-1
302 S cm2 mol-1
278 S cm2 mol-1
278 S cm2 mol-1
In a cell that utilises the reaction Zn(s) + 2H+ (aq) → Zn2+(aq) + H2(g) addition of H2SO4 to cathode compartment, will
lower the E and shift equilibrium to the left
increases the E and shift equilibrium to the left
increase the E and shift equilibrium to the right
increase the E and shift equilibrium to the right
The 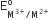 values for Cr, Mn, Fe and Co are – 0.41, +1.57, + 0.77 and +1.97 V respectively. For which one of these metals the change in oxidation state form +2 to +3 is easiest?
values for Cr, Mn, Fe and Co are – 0.41, +1.57, + 0.77 and +1.97 V respectively. For which one of these metals the change in oxidation state form +2 to +3 is easiest?
Cr
Co
Fe
Fe
How long (approximate) should water be electrolysed by passing through 100 amperes current so that the oxygen released can completely burn 27.66 g of diborane? (Atomic weight of B = 10.8 u)
1.6 hours
6.4 hours
0.8 hours
3.2 hours
D.
3.2 hours
According to the balanced equation:
27.66 g B2H6 i.e. 1 mole B2H6 requires 3 moles of O2. Now, this oxygen is produced by electrolysis of water.
1 mole O2 is produced by 4 F charge
therefore, 3 mole O2 will be produced by 12 F charge
hence, Now applying
Q = It
12 x 96500 C = 100 x t (s)
t = 3.2
