 Short Answer Type
Short Answer Type Long Answer Type
Long Answer TypeWhat are the reactions involved for ozone layer depletion in the stratosphere?
The ozonosphere region i.e. presence of ozone layer in the upper atmosphere prevents the harmful U.V. radiations from reaching our earth. But ozone depleting substances (ODS) are being released in the atmosphere by high-flying jets and rockets. These are converting ozone into oxygen. Actually, two types of compounds like nitric oxide and chlorofluorocarbons are responsible for depleting the ozone layer and creating a hole in it.
(i) Nitric oxide as ozone depleting substance: It is produced:
(a) at the ground level due to human activity or natural sources
or
(b) in large amounts in the exhaust gases by the engine of supersonic transport planes.
This NO is introduced directly into the stratosphere. NO reacts with ozone thereby decreasing the concentration of ozone and forms NO2.
NO2 then reacts with oxygen atoms available in the stratosphere (due to the decomposition of ozone and oxygen) producing back NO.
Thus no NO is consumed but O3 gets depleted.
(ii) chlorofluorocarbons (Freons) as ozone depleting substance: These are produced from:
(a) aerosol sprays in which they function as propellants and
(b) refrigerating equipment in which they act as coolants.
These are introduced in the stratosphere where they first undergo photochemical decomposition to give chlorine atoms.

The reactive chlorine atoms then destroy the ozone layer through the following sequence of reactions: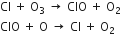
It has been found that one molecule of CFC can destroy one lakh O3 molecules in the stratosphere. Due to depletion of ozone, a large hole has been created in the ozone layer.
 Short Answer Type
Short Answer Type Long Answer Type
Long Answer Type Short Answer Type
Short Answer Type Long Answer Type
Long Answer TypeWrite down the reactions involved during the formation of photochemical smog.
Or
Discuss the mechanism of photochemical smog formation.
