 Short Answer Type
Short Answer Type Long Answer Type
Long Answer Type
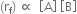
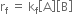



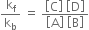
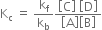
 is called equilibrium constant and has a constant value at a given temperature. Similarly, for a reaction
is called equilibrium constant and has a constant value at a given temperature. Similarly, for a reaction 

 Short Answer Type
Short Answer TypeHow is equilibrium constant expressed when the reaction is carried in the gaseous phase ?
 Long Answer Type
Long Answer Type Short Answer Type
Short Answer TypeWrite the expression for the equilibrium constant Kc for each of the following reactions:![]()
