 Short Answer Type
Short Answer Type Long Answer Type
Long Answer Type Short Answer Type
Short Answer TypeHow is equilibrium constant expressed when the reaction is carried in the gaseous phase ?
 Long Answer Type
Long Answer TypeUnder what conditions:
(i) Kp = Kc (ii) Kp > Kc (iii) Kp< Kc
We know, 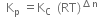
(i) 
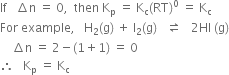
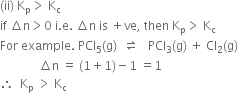
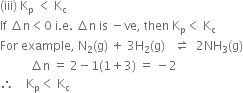
 Short Answer Type
Short Answer TypeWrite the expression for the equilibrium constant Kc for each of the following reactions:![]()
