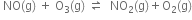Short Answer Type
Short Answer TypeWrite the expression for the equilibrium constant Kc for each of the following reactions:![]()
Write the expression for the equilibrium constant Kc for each of the following reactions:
Write the expression for the equilibrium constant Kc for each of the following reactions:![]()
Write the expression for the equilibrium constant Kc for each of the following reactions:![]()
 Long Answer Type
Long Answer TypeIt has been found that the pH of a 0.01 M solution of an organic acid is 4.15. Calculate the concentration of the anion, the ionisation constant of the acid and its pKa.
 Short Answer Type
Short Answer Type Long Answer Type
Long Answer Type(i) The equilibrium constant has a definite value for every chemical reaction at a given temperature. However, it varies with the change in temperature.
(ii) Its value is not influenced by the change in the concentration of reactants and products.
(iii) It is not affected by the presence of a catalyst.
(iv) The equilibrium constant for the forward reaction is the inverse of the equilibrium constant for the backward reaction.
For 

and for


Clearly

(v) The value of K tells us the extent to which the forward or backward reaction has taken place. The Greater value of Kc and Kp means that the reaction has proceeded to a greater extent in the forward direction.
(vi) The value of K changes if the coefficient of various species in the equation representing equilibrium are multiplied by the same number.
For example,


However, if we write 
Then 
 Short Answer Type
Short Answer Type