 Short Answer Type
Short Answer TypeA mixture of ![]() with molar concentration
with molar concentration ![]()
![]() and
and ![]() respectively was prepared at 500 K. At this temperature the value of
respectively was prepared at 500 K. At this temperature the value of ![]() for reaction
for reaction ![]() Predict whether at this state the concentration of
Predict whether at this state the concentration of ![]() will increases or decrease.
will increases or decrease.
The value of ![]() for the reaction
for the reaction ![]() At a given time the composition of reaction mixture is
At a given time the composition of reaction mixture is ![]() In which direction the reaction will proceed?
In which direction the reaction will proceed?
A mixutre of 1.57 mol of N2, 1.92 mol of H2 and 8.13 mol of NH3 is introduced into a 20L reaction vessel at 500K. At this temperature, the equilibrium constant, Kc, for the reaction![]() is
is ![]() . Is the reaction mixture at equilibrium? If not, what is the direction of the net reaction?
. Is the reaction mixture at equilibrium? If not, what is the direction of the net reaction?
The reversible reaction is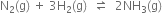
Concentration ratio,
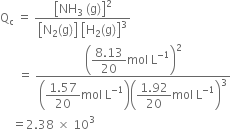
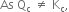 the reaction mixture is not in equilibrium.
the reaction mixture is not in equilibrium.
As Qc > Kc, the net reaction will be in the backward direction.
What is Kc for the following equilibrium when the equilibrium concentration of each substance
is: ![]()
![]()
 Long Answer Type
Long Answer Type