 Short Answer Type
Short Answer TypeA mixture of ![]() with molar concentration
with molar concentration ![]()
![]() and
and ![]() respectively was prepared at 500 K. At this temperature the value of
respectively was prepared at 500 K. At this temperature the value of ![]() for reaction
for reaction ![]() Predict whether at this state the concentration of
Predict whether at this state the concentration of ![]() will increases or decrease.
will increases or decrease.
The value of ![]() for the reaction
for the reaction ![]() At a given time the composition of reaction mixture is
At a given time the composition of reaction mixture is ![]() In which direction the reaction will proceed?
In which direction the reaction will proceed?
A mixutre of 1.57 mol of N2, 1.92 mol of H2 and 8.13 mol of NH3 is introduced into a 20L reaction vessel at 500K. At this temperature, the equilibrium constant, Kc, for the reaction![]() is
is ![]() . Is the reaction mixture at equilibrium? If not, what is the direction of the net reaction?
. Is the reaction mixture at equilibrium? If not, what is the direction of the net reaction?
What is Kc for the following equilibrium when the equilibrium concentration of each substance
is: ![]()
![]()
The reversible reaction is
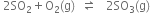
The equilibrium constant Kc is given by
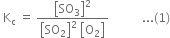
Putting the values in expression (1), we have
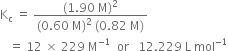
 Long Answer Type
Long Answer Type