 Long Answer Type
Long Answer TypeWhat is the effect of adding an inert gas(say He or N2):
(i) at constant volume and
(ii) at the constant pressure on the following equilibrium:![]()
The given dissociation equilibrium is


(i) Adding inert gas at constant volume. If an inert gas like helium or nitrogen etc. is added to a system at equilibrium at constant volume, then the total pressure increases. However, there will be no change in the position of equilibrium, even though the pressure has changed. This is because the concentrations of reactants and products (number of moles/volume) will not change. Hence the values of concentrations will continue to satisfy the equilibrium law. Hence the state of equilibrium will remain unaffected.
(ii) Adding inert gas at constant pressure. When an inert gas is added to a system at equilibrium keeping the pressure constant, the volume of the system increases. This results in a decrease in a number of moles of reactants per unit volume. According to Le-Chatelier’s principle, the equilibrium shifts in a direction in which there is an increase in the number of moles of gases. Hence the equilibrium shifts towards the forward direction where the number of moles of gases increases. In other words, more PCl5 dissociates to give PCl3 and Cl2. Hence dissociation of PCl5 increases with the addition of an inert gas.
 Short Answer Type
Short Answer Type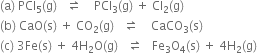
 Long Answer Type
Long Answer TypeWhich of the following reactions will get affected by increasing the pressure? Also, mention whether the change will cause the reaction to go into forward or backward direction?
 Short Answer Type
Short Answer Type Long Answer Type
Long Answer Type Short Answer Type
Short Answer TypeDescribe the effect of:
(a) addition of H2
(b) addition of CH3OH
(c) removal of CO
(d) removal of CH3OH
on the equilibrium of the reaction:
![]()
 Long Answer Type
Long Answer Type