 Short Answer Type
Short Answer TypeFind out the value of Kc for each of the following equilibria from the value of Kp:
CaCO3 (s) ![]() CaO(s) + CO2(g); Kp = 167 at 1073 K
CaO(s) + CO2(g); Kp = 167 at 1073 K
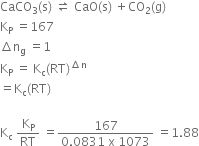
 Long Answer Type
Long Answer TypeOne of the reaction that takes place in producing steel from iron ore is the reduction of iron (II) oxide by carbon monoxide to give iron metal and CO2.![]()
What are the equilibrium partial pressures of CO and CO2 at 1050 K if the initial pressure are: ![]()
Bromine monochloride, BrCl decomposes into bromine and chlorine and reaches the equilibrium:
![]()
for which ![]() If initially pure BrCl is present at a concentration of
If initially pure BrCl is present at a concentration of ![]() what is its molar concentration in the mixture at equilibrium?
what is its molar concentration in the mixture at equilibrium?
At 1127 K and 1 atm pressure, a gaseous mixture of CO and CO2 in equilibrium with solid carbon has 90.55% CO by mass
![]()
Calculate Kc for this reaction at the above example.
 Short Answer Type
Short Answer Type