 Short Answer Type
Short Answer Type Long Answer Type
Long Answer TypeDiscuss the Lowry Bronsted concept of acids and bases.
Or
Discuss the protonic concept of acids and bases.
A species formed by adding a proton to Bronsted base is called conjugate acid and a species formed by losing a proton from Bronsted acid is called conjugate base. 
Thus, every acid has its conjugate base and every base has its conjugate acid. Consider a reaction between HCl and H2O.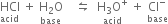
In the forward reaction, HCl donates a proton to water.
∴ HCl is an acid and H2O is a base.
In the backward reaction, H3O+ ion donates a proton to Cl– ion.
∴ H3O+ is an acid and Cl– is a base.
Thus Cl– ion is a conjugate base of HCl and H3O+ is the conjugate acid of base H2O. Such pairs of substances which differ from one another by a proton are known as conjugate acid-base pairs. Thus any acid-base reaction really involves two acids and two bases, forming conjugate pairs.
 Short Answer Type
Short Answer TypeState the formula and name of conjugate acid of the following bases:
(i) ![]() (ii) NH3
(ii) NH3
(iii) CH3COO– (iv) HS–
