 Short Answer Type
Short Answer TypeWrite the conjugate acids for the following Bronsted bases: ![]() , NH3 and HCOO–.
, NH3 and HCOO–.
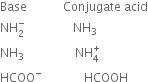
The species: ![]() can act both as Bronsted acids and bases. For each case give the corresponding conjugate acid and base.
can act both as Bronsted acids and bases. For each case give the corresponding conjugate acid and base.
 Long Answer Type
Long Answer TypeAll Arrhenius acids are Bronsted acids while all Arrhenius bases arc not Bronsted bases. Discuss.
 Short Answer Type
Short Answer Type Long Answer Type
Long Answer TypeDiscuss in brief Lewis concept of acids and bases.
Or
Discuss the electronic concept of acids and bases.
 Short Answer Type
Short Answer Type