 Short Answer Type
Short Answer TypeCalculate the degree of hydrolysis of 0.015 M solution of NH4Cl. Given Kb for NH4OH is 1·8 × 10–5, Kw = 10–14at 25°C.
 Long Answer Type
Long Answer TypeShow with an example how buffer solution resists the action of acid or base towards change in pH.
Or
Discuss the buffer action of:
(i) acidic buffer
(ii) basic buffer.
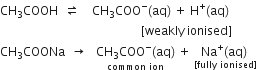


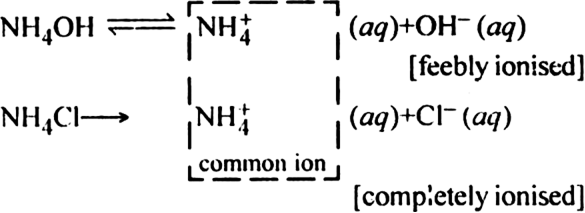
Ionisation of NH4OH is suppressed by NH4 + ions [common ion effect].
There will be a large concentration of NH4+ ions, Cl– ions and NH4OH molecules.
(i) When a few drops of acid (say HCl) are added, the H+ ions of the added acid combine with OH- ions of-NH4OH (of buffer) to form feebly ionised water. Thus no change in pH occurs.
According to Le Chatelier’s principle, the above reaction results in the greater dissociation of NH4OH to restore the original concentration of OH– ions.
(ii) When a few drops of a base (say NaOH) are added to the buffer, the OH– ions of added base combine with NH4+ ions to form unionised NH4OH.
As the concentration of OH– ions does not increase, the pH value remains unchanged.
In this buffer, reserve acidity is due to NH4+ ions and reserve alkalinity is due to NH4OH molecules.
Calculate the pH of:
(i) an acidic buffer mixture
(ii) a basic buffer mixture.
Or
Derive Henderson’s equation for an acidic and basic buffer mixture.
Or
Derive the following equation for the pH of an acidic buffer:![]()
 Short Answer Type
Short Answer Type Long Answer Type
Long Answer TypeDescribe Ostwald’s theory of acid-base indicators.
Or
How does Ostwald’s theory explain the colour change of:
(i) Phenolphthalein
(ii) Methyl orange in acid-base titrations?
 Short Answer Type
Short Answer TypeHow does the concept of solubility product help in finding out the solubility of sparingly soluble salts?
