 Short Answer Type
Short Answer TypeCalculate the solubility of lead iodide in water at 298 K.¬†(Ksp¬†of PbI2¬†= 7¬∑1√ó10‚Äď9).
 Long Answer Type
Long Answer TypeDetermine the solubility of silver chromate at 298K. Given that¬†Ksp¬†for Ag2CrO4¬†= 1¬∑1 √ó 10‚Äď12. Also determine the molarities of each ion.¬†

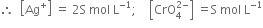
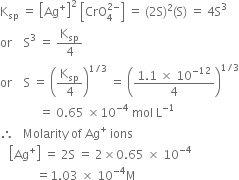
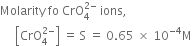
The solubility of Sr(OH)2 at 298K is 19·23g/L of the solution. Calculate the concentration of strontium and hydroxyl ions and pH of the solution. 
 Short Answer Type
Short Answer TypeHow many moles of AgBr¬†(Ksp¬†= 5 √ó 10‚Äď13¬†mol2¬†L‚Äď2) will dissolve in a 0¬∑01 M NaBr solution (NaBr is completely dissociated into Na+ and Br- ion).
