 Long Answer Type
Long Answer TypeIf 20 ml of 1·5 × 10–5 M BaCl2 solution is mixed with 40 ml of 0·9 × 10–5MNa2SO4 solution, will a precipitate get formed? Ksp for BaSO4 = 0·1 × 10–10.
If 25.0 cm3 of 0.50 M - Ba(NO3)2 are mixed with 25·0 cm3 of 0·0220M-NaF, will any BaF2 precipitate [Ksp of Ba F2 is 1·7 × 10–6 at 298 K.]
Equal volumes of 0.002M solutions of sodium iodate and cupric chlorate are mixed together. Will it lead precipitation of copper iodate? (For cupric iodate Ksp = 7·4 × 10–8).
 Short Answer Type
Short Answer TypeThe concentration of sulphide ion in 0·1M HCl solution saturated with hydrogen sulphide is 1·0 × 10–19M. If 10 mL of this solution is added to 5 mL of 0·04M solution of FeSO4, MnCl2, ZnCl2 and CaCl2, in which solutions precipitation will take place? Given Ksp for FeS = 6·3 × 10–18, MnS = 2·5 × 10–13, ZnS =1·6 × 10–24and CdS = 8·0 × 10–27.
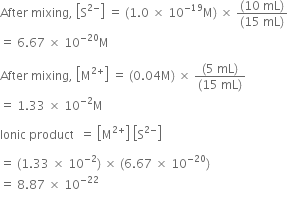
 Long Answer Type
Long Answer TypeThe values of Ksp of two sparingly soluble salts Ni(OH)2 and AgCN are 2·0 × 10–15 and 6 × 10–17 respectively. Which salt is more soluble ? Explain
The solubility product constant of Ag2CrO4 and AgBr are 1·1 × 10–12 and 5·0 × 10–13 respectively. Calculate the ratio of the molarities of their saturated solutions.
 Short Answer Type
Short Answer TypeFlow will you purify an impure sample of sodium chloride containing water soluble impurities?
