 Short Answer Type
Short Answer Type Long Answer Type
Long Answer Type Short Answer Type
Short Answer TypeGive reason: The precipitation of Mg(OH)2 is prevented by the addition of NH4Cl prior to the addition of NH4OH. But its precipitation by NaOH is not prevented by the prior addition of NaCl.
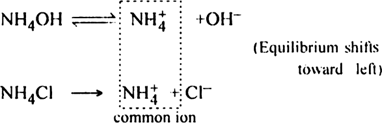
Due to common ion effect, NH4ions suppress the ionisation of NH 4OH. The low concentration of OH– ions is not sufficient for the ionic product [Mg2+][OH-]2 to exceed the solubility product of Mg(OH–)2. Hence it is not precipitated.
On the other hand, NaOH is a strong base and ionises almost completely. Its dissociation is not much affected by the common ion (Na+) from NaCl. Thus the ionic product [Mg2+][OH–]2 exceeds the solubility product value of Mg(OH)2. Hence Mg(OH)2 gets precipitated.
In which of the following, silver chloride will dissolve more:
(i) Pure water
(ii) 0·1M AgNO3 solution?
 Multiple Choice Questions
Multiple Choice QuestionsThe equilibrium constant at 298 K for a reaction A+B ⇌ C+D is 100. If the initial concentration of all the four species were 1 M each, then equilibrium concentration of D (in mol L−1 ) will be:
0.818
1.818
1.182
1.182
For the reaction,
if Kp = Kc (RT)x where the symbol has usual meaning then the value of x is (assuming ideality)
-1
-1/2
1/2
1/2
The species which can best serve as an initiator for the cationic polymerization is
LiAlH4
HNO3
AlCl3
AlCl3
The equilibrium constant (Kc) for the reaction, N2(g) + O2 (g) → 2NO (g) at temperature T is 4 x 10-4. The value of Kc for the reaction  at the same temperature is
at the same temperature is
0.02
2.5 x 102
4 x 10-4
4 x 10-4
