 Multiple Choice Questions
Multiple Choice QuestionsAmong the following acids which has the lowest pKa value?
CH3COOH
HCOOH
(CH3)2COOH
(CH3)2COOH
The conjugate base of H2PO4- is
PO43-
HPO42-
H3PO4
H3PO4
B.
HPO42-
H3PO4 is a tribasic acid, thus ionising in three steps.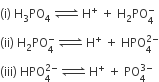
What is the equilibrium expression for the reaction P4(s) +5O2(g)⇌ P4O10(s)?
Kc = [P4O10] / P4] [O2]5
Kc = 1/[O2]5
Kc = [O2] 5
Kc = [O2] 5
The equilibrium constant for the reaction N2(g) + O2(g) ⇌ 2NO(g) at temperature T is 4×10-4. The value of Kc for the reaction NO(g) ⇌ 1/2N2(g) + 1/2O2(g) at the same temperature.
2.5×102
0.02
4 x 10-4
4 x 10-4
An alkali is titrated against an acid with methyl orange as indicator, which of the following is a correct combination?
| Base | Acid | End Point |
| Strong | Strong | Pink to colourless |
| Base | Acid | End Point |
| Weak | Strong | Colourless to pink |
| Base | Acid | End Point |
| Strong | Strong | Pinkish Red to Yellow |
| Base | Acid | End Point |
| Weak | Strong | Yellow to Pinkish Red |
Which of the following are Lewis acids?
BCl3 and AlCl3
PH3 and BCl3
AlCl3 and SiCl4
PH3 and SiCl4
Dissolving NaCN in de-ionised water will result in a solution having
pH < 7
pH = 7
pOH = 7
pH > 7
