 Multiple Choice Questions
Multiple Choice QuestionsA current strength of 9.65 A is passed through excess fused AlCl3 for 5 h. How many litres of chlorine will be liberated at STP? (F = 96500 C)
2.016
1.008
11.2
20.16
The colour of the soltuion/ precipitate obtained in the elemental analysis of an organic compound and the molecule/ion responsible for the colour are given below. Choose the incorrectly matched pair.
Prussian blue - Fe4[Fe(CN)6]3.xH2O
Yellow - (NH4)2MoO4
Violet colour - [Fe(CN)5NOS]4-
Blood red colour - [Fe(SCN)]2+
Among the following amines, which one has the highest pK, value in aqueous solution?
Methanamine
N, N-dimethylaniline
Ethanamine
Benzenamine
In the following equilibrium reaction,
2A B + C
the equilibrium concentration of A, B and C are 1 × 10-3 M, 2 × 10-3 M and 3 × 10-3 M respectively at 300 K. The value of Kc for this equilibrium at the same temperature is
6
36
Which one of the following is the correct statement?
HCO is the conjugate base of CO
NH is the conjugate acid of NH3
H2SO4 is the conjugate acid of HSO
NH3 is the conjugate base of NH
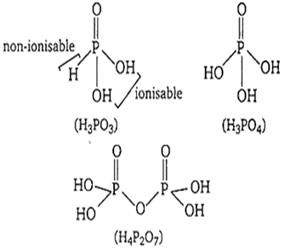
Choose the weak monobasic acid, among the following
H3BO3
H3PO3
H3PO4
HNO3
A.
H3BO3
H3BO3 is a weak Lewis monobasic acid, because it can only accept a pair of electron. None of its hydrogens are ionisable.
In the reaction H2S+ H2O2 → S+ 2H2O
H2S is an acid and H2O2 is a base
H2S is a base and H2O2 is an acid
H2S is an oxidising agent and H2O2 is a reducing agent
H2S is an reducing agent and H2O2 is a oxidising agent
The solubility product (Ks) of the following compounds are given at 25°C
| Compounds |
Ksp |
| AgCl | 1.1 × 10-10 |
| AgI | 1.0 × 10-16 |
| PbCrO4 | 4.0 × 10-14 |
| Ag2CO3 | 8.0 × 10-12 |
The most soluble and least soluble compounds are
AgCl and PbCrO4
AgI and Ag2CO3
AgCl and Ag2CO5
Ag2CO3 and AgI
Four moles of PCl5 are heated in a closed 4 dm3 container to reach equilibrium at 400 K. At equilibrium 50% of PCl5 is dissociated. What is the value of Kc for the dissociation of PCl5 into PCl3 and Cl2 at 400 K?
0.50
1.00
1.25
0.05
A weak monobasic acid is 1% ionized in 0.1 M solution at 25°C. The percentage of ionisation in its 0.025 M solution is
1
2
3
4
