 Long Answer Type
Long Answer TypeExplain different methods of reduction of the roasted/calcined ore to the metallic form.
The crude metal is refined by one or more of the following techniques:
(i) Liquation: Easily fusible metals like Hg, Sn, etc. are heated in a sloping hearth of a reverberatory furnace. The fusible metal melts and flows down in pure form, leaving behind the infusible impurities called drop.
(ii) Fractional distillation: Volatile metals like Hg, Zn etc. are submitted to distillation when pure metal distills over, leaving behind non-volatile impurities.
(iii) Polling: It is a process of stirring the hot molten anode metal with green logs of wood. The wood gases like methane and other hydrocarbons produced from green logs reduce metal oxide impurity to the metallic form. Moreover, during stirring large quantity of air is absorbed by the molten metal, oxidises easily oxidisable impurities. The oxidised impurities escape either as vapour or form scum over molten metal, which can be scooped out by perferated ladle.
(iv) Electrolytic refining: In this method, large block of impure metal is made anode in an electrolytic cell and a thin sheet of pure metal is made the cathode. Suitable metal salt solution is made an electrolyte. On passing current, pure metal deposits on the thin cathode sheet. While the more electropositive pure metal deposits on the thin cathode sheet while the more electropositive metallic impurities are left in solution and noble metal impurities settle below the anode as anode mud. Most of the metals are refined by this method.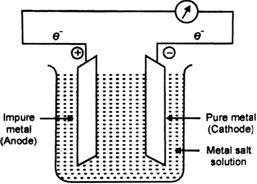
Fig. Electrolytic purification of metal.
(v) Zone refining: This method is used when highly pure metal is required for the purpose as semi-conductors in transistors and solar batteries.
It is based on the fact that melting point of a substance is lowered when impurities are present. Consequently, when an impure molten metal is cooled, crystals of pure metal are solidified and the impurities remains in molten metal.
The process consists in casting the impure metal in the form of a bar. A circular heater is fitted around this bar and the circular heater is moved slowly longitudinally from one end to the other. At the heated zone, the bar melts and as the heater moves on, pure metal crystallizes, while impurities pass into the adjacent molten part.
In this way, the impurities are swept from one end of the bar to the other. By repeating the process, ultra pure metal of silicon, germanium etc. are obtained.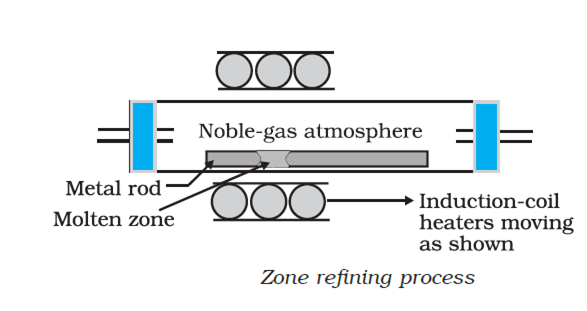
Fig. Zone Refining.
 Fill In the Blanks
Fill In the BlanksIn the metallurgical process for the electrorefining of the metal the anode is made of _________ metal.
In the Bessemer converter during the extraction of copper, the substance responsible for forming blister is ___________.
During the extraction, the metallic silver is precipitated by the addition of __________ to ________
