 Short Answer Type
Short Answer TypeArrange each set of compounds in order of increasing boiling points:
Bromomethane, Bromoform, Chloro-methane, Dibromomethane.
Alkyl halides containing same alkyl group,the boiling point increases with an increase in the atomic mass of the halgoen. Since the atomic mass bromine is greater than chlorine, the boiling point of bromomethane is higher than that of chloromethane. Also, for alkyl halides containing the same alkyl group, the boiling point inrease in as number of halide group increase. Therefore, the boiling point of dibromomethane is higher than chloromethane and bromomethane, but lower than bromoform. Such as,
Chloromethane<bromomethane<dibromomethane<bromoform.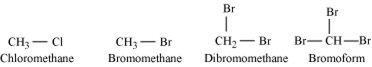
Arrange each set of compounds in order of increasing boiling points:
1-chloropropane, Isopropyl chloride, 1-chlorobutane.
Which alkyl halide from the following pairs would you expect to react more rapidly by an SN2 mechanism? Explain your answer.

