 Short Answer Type
Short Answer TypeArrange each set of compounds in order of increasing boiling points:
Bromomethane, Bromoform, Chloro-methane, Dibromomethane.
Arrange each set of compounds in order of increasing boiling points:
1-chloropropane, Isopropyl chloride, 1-chlorobutane.
Arrange each set of compounds in order of increasing boiling points:![]()
1-bromobutane is a primary alkyl halide whereas 2-bromobutane is secondary alkyl halide. The nucleophile approaching is more hindered in 2- bromobutane than in 1-bromobutane. Therefore, 1- bromobutane reacts more rapidly than 2- bromobutane by an SN2 mechainsm. 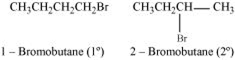
Which alkyl halide from the following pairs would you expect to react more rapidly by an SN2 mechanism? Explain your answer.

