 Short Answer Type
Short Answer TypeArrange each set of compounds in order of increasing boiling points:
Both of the alkyl halides are primary. However, the substituent CH3 is at a greater distance to the carbon atom linked to Br in 1- bromo-3-methylbutane than in 1-bromo-2-methylbutane. Therefore, the approaching nucleophile is less hindered in case of the 1-bromo-3-methylbutane than in case of the 1-bromo-2-methylbutane. Hence, 1-bromo-3-methylbutane reacts faster than the latter by SN2 mechanism.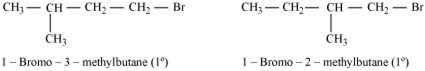
In the following pairs of halogen compounds, which compound undergoes faster SN1 reaction?
![]() and
and 
In the following pairs of halogen compounds, which compound undergoes faster SN1 reaction?![]()
Thionyl chloride is preferred in the preparation of chloro alkanes from alcohol. Give reason.
