 Short Answer Type
Short Answer TypeIn the following pairs of halogen compounds, which compound undergoes faster SN1 reaction?
![]() and
and 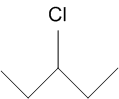
In the following pairs of halogen compounds, which compound undergoes faster SN1 reaction?![]()
Greater the stability of the carbocation, faster is the rate of SN1 reaction. Since secondary carbocation 2-chloroheptane is more stable than primary carbocation 1-chlorohexane. Hence SN2 reaction proceed via secondary cation such as 2-chloroheptane.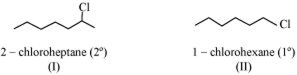
Thionyl chloride is preferred in the preparation of chloro alkanes from alcohol. Give reason.
