 Short Answer Type
Short Answer TypeArrange the following compounds in increasing order of SN1 , respectively.
(CH3)3CCI, C6H5(CH3)2Cl, CH3CH2CH2Cl
What are the structure of the possible isomes of dichloroethane? Which of them will have zero dipole movement?
Arrange the following alkyl halides in the decreasing order of SN2 reactivity.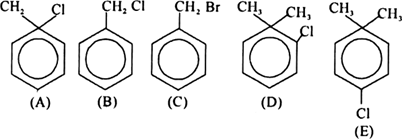
(B) and (C) are primary and are very reactive, but bromide is more reactive than chloride.
(D) and (E) are both secondary but nucleophile attack on (D) is hindered because of stearic hindrance by two —CH3 group.
(A) is tertiary and therefore not reactive under SN2 conditions ;
(C) > (B) > (E) > (D) > (A).
Give reasons:
Iodoform is obtained by the reaction of acetone with hypoidodide ion and not with iodide ion.
