 Short Answer Type
Short Answer TypeWhat happens when steam is passed through methane at 1273 K in the presence of nickel catalyst?
How is iodination of alkane shifted in the forward direction?
Iodination is extremely slow and reversible in nature.
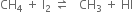
HI is a strong reducing agent and converts methyl iodide back to methane. In order to carry out the reaction in the forward direction. HI is destroyed with the help of an oxidising agent like iodic acid (HIO3), concentrated. HNO3 or mercuric oxide (HgO).
