 Long Answer Type
Long Answer TypeDiscuss:
(a) Nitration of an alkane.
(b) Sulphonation of alkane.
(a) Nitration of alkane: The process which involves the replacement of hydrogen atom of alkanes by nitro group (-NO2) is known as nitration of an alkane. It can be carried out in two ways:
(i) Liquid phase nitration; In this method higher alkane is heated with fuming HNO3 at 413 K under pressure.
(ii) Vapour phase nitration: Lower member of alkanes can be nitrated by vapour phase nitration i.e. by heating a gaseous mixture of hydrocarbon and nitric acid vapours at 673-773 K.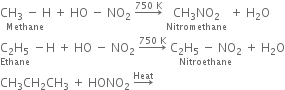
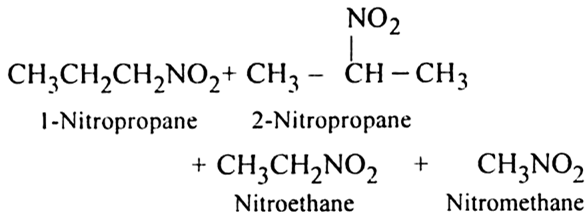
Mechanism of nitration:
The nitration of alkanes proceeds by the free radical mechanism.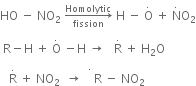
(b) Sulphonation of alkane: The process which involves the replacement of hydrogen atom of alkanes with a sulphonic acid group (-SO3H) is known as sulphonation of alkane.
It is carried out by heating higher alkanes (hexane or higher members) with fuming sulphuric acid.
Mechanism of sulphonation:
The sulphonation of alkanes proceeds by the free radical mechanism.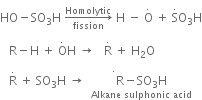
 Short Answer Type
Short Answer Type Long Answer Type
Long Answer Type Short Answer Type
Short Answer Type Long Answer Type
Long Answer TypeOut of staggered and eclipsed conformations of ethane, which is more stable and why ?
 Short Answer Type
Short Answer Type